Jacobio Pharma (1167.HK) has received IND (Investigational New Drug) approval of CD73 mAb JAB-BX102 from the Center for Drug Evaluation (the “CDE”) of China in March 24, 2022. Jacobio plans to initiate a Phase I clinical trial in patients with advanced solid tumours in China.
JAB-BX102 is a humanised monoclonal antibody that specifically inhibits the enzymatic activity of CD73. Previously, the IND for Phase I/IIa clinical trial of JAB-BX102 was approved by the Food and Drug Administration of the United States on October 2021.
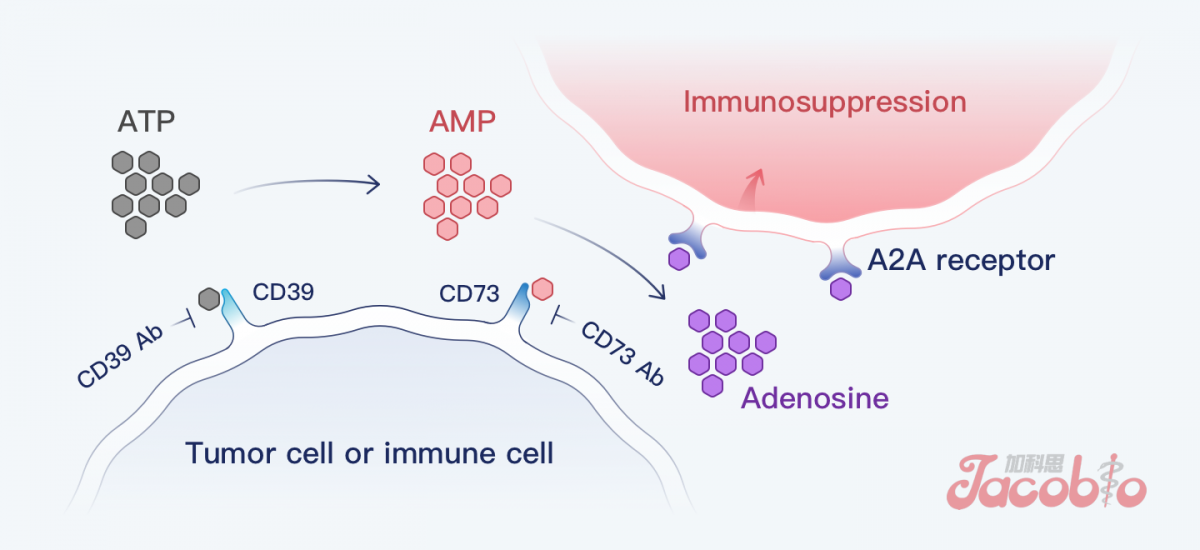
The mechanism of CD73
CD73 is an important target in the adenosine pathway, and to date, there has been no approved and marketed CD73 targeted drug globally. Preclinical data have shown that JAB-BX102 has the advantage of dose activity and has the potential to benefit patients with solid tumours.
Jacobio’s pipeline revolves around six signalling pathways in cancer: RAS, MYC, RB, I/O, cancer metabolic and P53, and aim to find unarguable targets in validated pathways. Currently, Jacobio has six clinical stage programs, both the SHP2 and Aurora A inhibitors are among the first three products in their categories in the world to have entered the clinical stage. Jacobio also has seven pre-clinical programs, three of them will submit IND applications in 2022, including JAB-24114 (undisclosed target in tumour metabolic pathway ), JAB-26744 (undisclosed target in I/O pathway), JAB-BX300 (undisclosed target in RAS).
Jacobio(1167.HK) is committed to providing more products and solutions to people's health. Our mission is to provide compelling innovations for creating a pipeline of life-changing medicines. Our vision is to become a global leader recognized for our impact in drug R&D together with our partners. The company's R&D centers are located in Beijing, Shanghai and MA, with a platform and expertise in developing allosteric inhibitors against protein tyrosine phosphatase, KRAS and transcriptional factors.